Bioxydyn offers MRI measurement of liver transporter fluxes using dynamic contrast-enhanced MRI (DCE-MRI), plus other imaging biomarkers, including relaxation time measurements, in the investigation of liver diseases.
Bioxydyn is the lead commercial partner in the IMI TRISTAN project, which is investigating the liver and lung toxicity of many drugs in use today with the goal of validating the use of imaging biomarkers to assess and predict the toxicity of potential medicines on the liver and lungs.
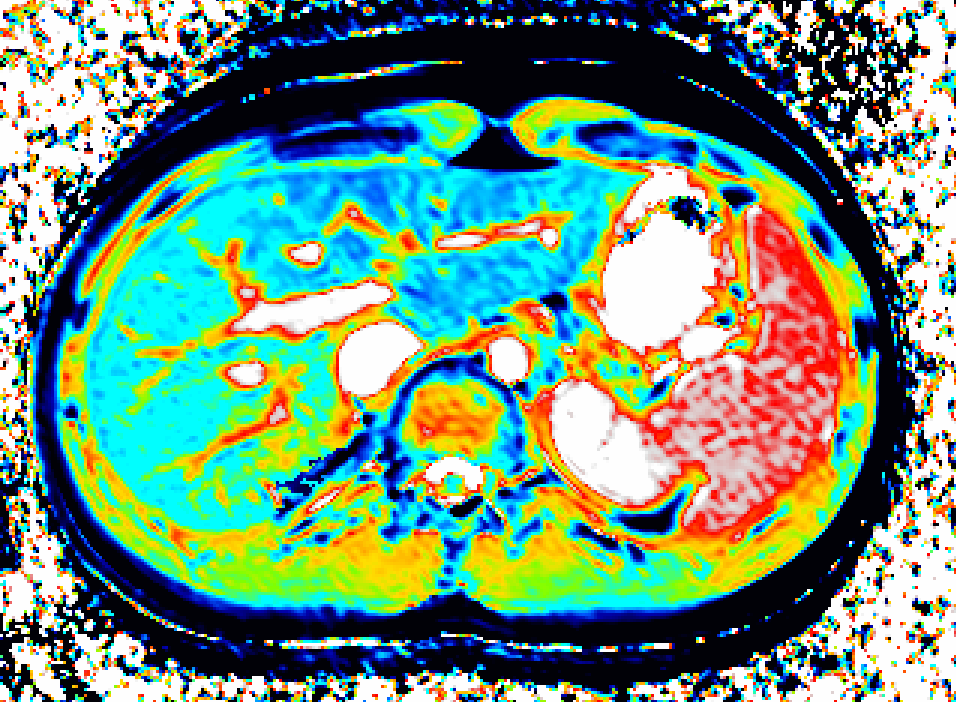
We use dynamic contrast-enhanced MRI (DCE-MRI) of gadoxetate to measure liver transporter function. We use relaxation time measurements to assess tissue status.
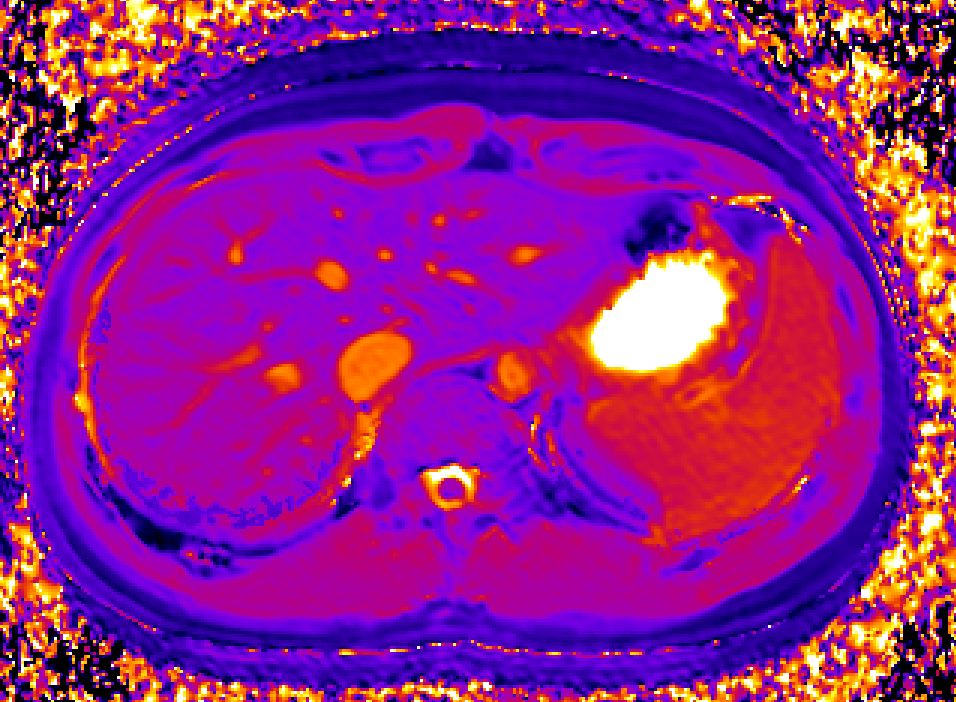
Drugs and other xenobiotics are commonly eliminated via the liver. Hepatocyte uptake and efflux involve multiple transporters with different specificities. If drug uptake is perturbed, it may be cleared less (or more) rapidly from the blood, leading to harmful overdosing (or lack of efficacy). Drug-Drug Interactions (DDI), where one drug inhibits or enhances the uptake flux of a second drug, hinder safe prescribing. If efflux is inhibited, harmful levels of drug may accumulate in the hepatocyte leading to drug- induced liver injury (DILI). Uptake and efflux kinetics cannot be unambiguously determined from blood levels, but imaging biomarkers provide specificity.
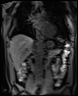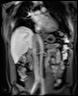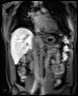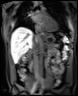
The TRISTAN consortium aimed to develop and validate gadoxetate-based DCE-MRI biomarkers for DILI and DDI. Drug developers and regulatory authorities are more likely to rely on such imaging biomarkers if they trust the acquisition, analysis and interpretation. For this reason we have developed a precise specification which has been accepted into FDA’s biomarker qualification programme (BQP): "the change, caused by investigational (perpetrator) drug, in gadoxetate (victim drug surrogate) hepatocyte uptake and efflux rate constants [∆k(he) and ∆k(bh), respectively] indicating potential for drug-drug interaction when above threshold" and the context is use is: "a safety (acute lack-of-harm) biomarker, employed in the development of investigational drugs (IDs) which are thought to carry an enhanced DDI risk because prior animal or in vitro human cell studies have indicated that the ID is a hepatic transporter inhibitor or inducer. ∆k(he) and ∆k(bh) would be used in early phase clinical drug development. An ID whose effect on either ∆k(he) or ∆k(bh) is not below-threshold would be prioritized for an early program of clinical DDI investigations".
Our platform of evidence includes a range of in vitro, rat, and human studies of the reproducibility of the assay and its response to multiple known transporter substrates.
Melillo N, Scotcher D, Kenna JG, Green C, Hines CDG, Laitinen I, Hockings PD, Ogungbenro K, Gunwhy ER, Sourbron S, Waterton JC, Schuetz G, Galetin A, Use of In Vivo Imaging and Physiologically-Based Kinetic Modelling to Predict Hepatic Transporter Mediated Drug-Drug Interactions in Rats. Pharmaceutics. 2023 Mar 10;15(3):.
Tadimalla S, Wilson DJ, Shelley D, Bainbridge G, Saysell M, Mendichovszky IA, Graves MJ, Guthrie JA, Waterton JC, Parker GJM, Sourbron SP, Bias, Repeatability and Reproducibility of Liver T(1) Mapping With Variable Flip Angles. J Magn Reson Imaging. 2022 Oct;56(4):1042-1052.
Waterton JC, Survey of water proton longitudinal relaxation in liver in vivo. MAGMA. 2021 Dec;34(6):779-789.
Berks M, Little RA, Watson Y, Cheung S, Datta A, O'Connor JPB, Scaramuzza D, Parker GJM, A model selection framework to quantify microvascular liver function in gadoxetate-enhanced MRI: Application to healthy liver, diseased tissue, and hepatocellular carcinoma. Magn Reson Med. 2021 Oct;86(4):1829-1844.
Ulloa JL, Stahl S, Yates J, Woodhouse N, Kenna JG, Jones HB, Waterton JC, Hockings PD, Assessment of gadoxetate DCE-MRI as a biomarker of hepatobiliary transporter inhibition. NMR Biomed. 2013 Oct;26(10):1258-70.
Banerji A, Naish JH, Watson Y, Jayson GC, Buonaccorsi GA, Parker GJ, DCE-MRI model selection for investigating disruption of microvascular function in livers with metastatic disease. J Magn Reson Imaging. 2012 Jan;35(1):196-203.